The Uranium Committee
The U.S. President appointed a committee, known as the ‘Advisory Committee on Uranium‘, consisting of Lyman James Briggs (director of the U.S. National Bureau of Standards) as chairman, Colonel K. F. Adamson of the Army Ordnance Department, and Commander G. C. Hoover of the Navy Bureau of Ordnance, and requested this committee to look into the problem. From June 1940 there was a technical sub-committee with Harold Clayton Urey (1934 Nobel Prize for Chemistry), Ross Gunn, George Pergram, Merle Anthony Tuve, Jesse Wakefield Beams, and Gregory Breit. The committee on uranium was the only committee that had official status up to the time of the organisation of the National Defence Research Committee in June 1940. The committee met very informally and included various additional scientific representatives in its meetings.
The first meeting of the Uranium Committee was on October 21, 1939 and included, besides the committee members, F. L. Mohler, Alexander Sachs, Leo Szilárd, Eugene Wigner, Edward Teller, and Richard Brooke Roberts. The result of this meeting was a report dated November 1, 1939, and transmitted to President Roosevelt by Briggs, Adamson, and Hoover. This report made eight recommendations, and specifically mentioned both atomic power and an atomic bomb as possibilities. It recommended procurement of 4 tons of graphite and 50 tons of uranium oxide for measurements of the absorption cross-section of carbon.
The first transfer of funds ($6,000) from the Army and Navy to purchase materials in accordance with the recommendation of November 1, 1939, is reported in a memorandum from Briggs to General Edwin Martin Watson (President Roosevelt’s aide) on February 20, 1940. The next meeting of the ‘Advisory Committee on Uranium’ was on April 28, 1940 and was attended by Sachs, Wigner, Pegram, Enrico Fermi, Szilárd, Briggs, Admiral Harold Gardiner Bowen, Colonel Adamson, and Commander Hoover.
The Army and Navy each gave $3,000 to National Bureau of Standards, which gave the money to Columbia University (i.e. Fermi), who used it to buy an ‘inordinate’ quantity of graphite. The official line was that the Army was only convinced to allocate these fund when it learned that the President “was interested in this project”. However the story is a bit different. It was Teller who had referred incidentally to the amount of money that could be spent profitably in the months ahead. Colonel Adamson noted that usually it took two wars to develop a new weapon, and it was morale that won wars, not arms. Wigner replied that if armaments were so unimportant a cut of 30% in the defence budget might be a good idea. Apparently Adamson snapped that “you’ll get your money”.
$6,000 today does not sound like very much, but at the time (February 1940) it reflected the importance attached to the Fermi-Szilárd pile experiments already underway at Columbia University. Building upon the work performed in 1934 demonstrating the value of moderators in producing slow neutrons, Fermi thought that a mixture of the right moderator and natural uranium could produce a self-sustaining chain reaction. Fermi and Szilárd increasingly focused their attention on carbon in the form of graphite. Perhaps graphite could slow down, or moderate, the neutrons coming from the fission reaction, increasing the probability of their causing additional fissions in sustaining the chain reaction. A pile containing a large amount of natural uranium could then produce enough secondary neutrons to keep a reaction going.
There was, however, a large theoretical gap between building a self-generating pile and building a bomb. Although the pile envisioned by Fermi and Szilárd could produce large amounts of power and might have military applications (powering naval vessels, for instance), it would be too big for a bomb. It would take separation of uranium-235 or substantial enrichment of natural uranium with uranium-235 to create a fast-neutron reaction on a small enough scale to build a usable bomb. While certain of the chances of success in his graphite power pile, Fermi, in 1939, thought that there was “little likelihood of an atomic bomb, little proof that we were not pursuing a chimera“.
By the time of this meeting two important new factors had come to light. First, it had been discovered that the uranium fission caused by neutrons of thermal velocities occurred in the uranium-235 isotope only. Second, it had been reported that a large section of the Kaiser Wilhelm Institute in Berlin had been set aside for research on uranium.

Fission may take place in any of the heavy nuclei after capture of a neutron. However, low-energy (slow, or thermal) neutrons are able to cause fission only in those isotopes of uranium and plutonium whose nuclei contain odd numbers of neutrons (e.g. U-233, U-235, and Pu-239). As seen above the fission cross-section increases dramatically (when competing with the capture cross-section) for thermal neutron (<0.025 eV) on U-235 than for higher energies (1/v dependence).
Within weeks a number of people, particularly Sachs, urged the importance of greater support and of better organisation. Their hand was strengthened by the Columbia results (as reported, for example, in a letter from Sachs to General Watson on May 15, 1940) showing that the carbon absorption was appreciably lower than had been previously thought and that the probability of carbon being satisfactory as a moderator was therefore considerable. Sachs was also active in looking into the question of uranium ore supply. On June 1, 1940, Sachs, Briggs, and Urey met with Admiral Bowen to discuss approaching officials of the Union Miniere of the Belgian Congo. Such an approach was made shortly afterwards by Sachs.
In fact Edgar Sengier, head of Union Miniere, had already ordered a shipment of 1,200 tons of high-grade ore from the Shinkolobwe stockpile in the Congo, via Portuguese West Africa to New York. Oddly enough at the time the U.S. government was not interesting in this stockpile, but it was ‘discovered’ when Standard Oil, working on the centrifuge process, opened negotiations. Thus, by an odd series of events, the U.S. found enough uranium ore, just when they needed it.
The general status of the problem was discussed by a special advisory group called together by Briggs at the National Bureau of Standards on June 15, 1940. This meeting was attended by Briggs, Urey, Merle Anthony Tuve, Wigner, Breit, Fermi, Szilárd, and Pegram. After full discussion, the recommendation of the group to the Uranium Committee was that funds should be sought to support research on the uranium-carbon experiment along two lines:-
- Further measurements of the nuclear constants involved in the proposed type of reaction.
- Experiments with amounts of uranium and carbon equal to about 1/5th to 1/4th of the amount that could be estimated as the minimum in which a chain reaction would sustain itself.
“It was estimated that about $40,000 would be necessary for further measurements of the fundamental constants and that approximately $100,000 worth of metallic uranium and pure graphite would be needed for the intermediate experiment”.
(Quote from memorandum of Pegram to Briggs, dated August 14, 1940)
In fact as far as I can see this money was used to purchase 4 tons of pure graphite and 50 tons of uranium oxide. The same meeting also decided that any large-scale uranium-carbon experiment should be supervised by the Army and Navy at one of their proving grounds. They also decided that isotope separation work should continue in the universities, and not on a secret basis.
As the Nazis were over running Belgium and looking towards France, there was increasing pressure for a research program with improved financial support and a more flexible organisation. The Uranium Committee had done its job, it had determined that the focus should be on both isotope separation and the chain reaction.
National Defence Research Committee
Before any decisions made at the last meeting of the Uranium Committee could be put into effect, the organisation of the National Defence Research Committee (NDRC) was announced in June 1, 1940, and President Roosevelt gave instructions that the Uranium Committee should be reconstituted as a subcommittee of the NDRC, reporting to Vannevar Bush (chairman, NDRC). The membership of this reconstituted Uranium Committee (or Section) was as follows: Briggs (Chairman), Pegram, Urey, Beams, Tuve, and Ross Gunn. In September 1941 Gunn and Tuve stood down, and the Committee was enlarged with the introduction of Breit, Samuel King Allison, Edward Uhler Condon, Henry DeWolf Smyth, and Lloyd Smith. On authorisation from Briggs, Breit consulted Wigner and Teller frequently although they were not members of the committee.
The National Defence Research Committee, with Vannevar Bush at its head, reorganised the Uranium Committee into a scientific body and eliminated military membership. Not dependent on the military for funds, as the Uranium Committee had been, the National Defence Research Committee would have more influence and more direct access to money for nuclear research. In the interest of security, Bush barred foreign-born scientists from committee membership and blocked the further publication of articles on uranium research. Retaining program responsibilities for uranium research in the new organisation as setup (among the National Defence Research Committee’s early priorities were studies also on radar, proximity fuses, and anti-submarine warfare), the Uranium Committee recommended that isotope separation methods and the chain reaction work continue to receive funding for the remainder of 1940. Bush approved the plan and allocated the funds.
Through to December 1941, a number of contracts were let. Their number and total amount grew gradually. Urey began to work on isotope separation by the centrifuge method under a Navy contract in the fall of 1940. Other contracts were granted to Columbia University, Princeton University, Standard Oil Development Company, Cornell University, Carnegie Institution of Washington, University of Minnesota, Iowa State College, John Hopkins University, National Bureau of Standards, University of Virginia, University of Chicago, and University of California in the course of the winter and spring of 1940-1941, until by November 1941 the total number of projects approved was sixteen, totalling about $300,000 ($100,000 for isotope separation and $140,000 for the chain reaction).
The Uranium Committee as formed in the summer of 1940 continued substantially unchanged until the summer of 1941. At that time the main committee was somewhat enlarged and subcommittees formed on isotope separation (Urey), theoretical aspects (Fermi, Szilárd), power production (Fermi, Szilárd) and heavy-water (Urey). It was thereafter called the Uranium Section or the S-l Section of NDRC.
Even today there are still inconsistencies across different ‘official’ histories leading up to the Manhattan Project. Some reports mention these ‘subcommittees’, others don’t. In some reports it is mentioned that Uranium Committee became a Section (S-1) in the summer 1941. It is said that dropping the word uranium was, at least in part, for security reasons. Other reports mention that in June 1941 Roosevelt created the Office of Scientific Research and Development (OSRD). The NDRC fell under the new OSRD, and Vannevar Bush became its director, with James Bryant Conant as head of NDRC. The Uranium Committee became one of the ‘sections’ under the OSRD, however it was only in January 1942 that the section was reorganised and renamed Section ‘S-1’. It was in fact removed from the NDRC and made an independent organisation within the OSRD. By June 1942 the chair of Section S-1 increasingly had the task of managing the relationship between OSRD and what was fast becoming an army project.
We have to remember that Germany invaded Norway in early April, 1940, securing control of the Norsk Hydro plant, the only large facility in the world producing heavy-water (deuterium oxide). This plant was sabotaged by the Norwegians to prevent the Germans acquiring the heave water. Harold Urey discovered the isotope deuterium in 1931 and was later able to concentrate it in water. Heavy-water is an alternative neutron moderator to graphite. Because they do not require uranium enrichment, heavy-water reactors are more of a concern for nuclear proliferation. The breeding and extraction of plutonium can be a relatively rapid and cheap route to building a nuclear weapon, as chemical separation of plutonium from fuel is easier than isotopic separation of uranium-235 from natural uranium.
In the spring of 1941, Briggs, feeling that an impartial review of the problem was desirable, requested Bush to appoint a review committee. Bush then formally requested Frank Baldwin Jewett, president of the National Academy of Sciences, to appoint such a committee. Jewett complied, appointing Compton (chairman), William David Coolidge, Ernest Lawrence, John Clarke Slater, John Hasbrouck Van Vleck, and Bancroft Gherardi Jr. This committee was instructed to evaluate the military importance of the uranium problem and to recommend the level of expenditure at which the problem should be investigated.
Again there are substantial discrepancies in different ‘histories’ about the members of this review. Other membership lists don’t mention Slater and Gerard Jr. as members, but do mention Oliver Ellsworth Buckley, Lewis Warrington Chubb, Warren Kendall Lewis, George Bogdanovich Kistiakowsky, and Robert Sanderson Mulliken.
Arthur Holly Compton (1892-1962) won the Nobel Prize in Physics in 1927 for the discovery of the Compton effect, thus demonstrating the particle nature of electro-magnetic radiation. He was leader of the Metallurgical Laboratory in Chicago, and later was in charge of plutonium production for the bomb.
Ernest Orlando Lawrence (1901-1958) won the Nobel Prize in Physics in 1939 for the invention of the cyclotron, and was responsible for isotope separation and uranium enrichment with the calutrons.
Pegram summarised progress in a report dated February 15, 1941 showing that the main progress had been made on understanding the slowing down of neutrons in graphite, on the number of neutrons emitted in fission, and on the design and size of lattices.
The slowing down of neutrons in graphite was investigated by studying the intensity of activation of various metal foils (rhodium, indium, iodine) placed at various positions inside a rectangular graphite column. With a source of neutrons placed at the bottom and using cadmium screens (cadmium having a very large absorption cross-section for thermal neutrons) the effects of resonance and thermal neutrons could be measured separately. These results, coupled with theoretical studies of the diffusion of thermal neutrons, laid a basis for future calculations of the number of thermal and resonance neutrons to be found at any point in a graphite mass of given shape when a given neutron source is placed at a specified position within or near the graphite. The experiments on slowing down neutrons showed that high-energy neutrons such as those from fission were practically all reduced to thermal energies after passing through 40 cm or more of graphite. A piece of uranium placed in a region where thermal neutrons were present absorbed the thermal neutrons and as fission occurred re-emitted fast neutrons, which were easily distinguished from the thermal neutrons. By a series of measurements with and without uranium present and with various detectors and absorbers, it was possible to get a value for the constant 𝜼, the number of neutrons emitted per thermal neutron absorbed by uranium. This is not the number of neutrons emitted per fission ƙ, but is somewhat smaller than that number since not every absorption causes fission.
The Fission of Uranium
In the last paragraph we have introduced a number of new physical concepts that need clarification within the context of the time. Research on the physics and chemistry needed to build an atomic bomb was substantial and it is impossible to even imagine summarising it all on one webpage, so I’ve decided here and there to focus on one or other key topic, and the first topic is the work of Fermi on the fission of uranium.
Fermi received the 1938 Nobel Prize in Physics, and after receiving his prize he continued on to New York where he applied for permanent residency. In early 1939 he took up a post as professor of physics at Columbia University. He more or less started where he had left off, looking at the fission process of the uranium nucleus by neutron bombardment.
Otto Hahn and Fritz Strassmann had found chemical evidence for the splitting of the uranium nucleus into two approximately equal parts. Lise Meitner and Otto Robert Frisch had suggested that the release of energy could be as much as 200 MeV. Fermi and his team started by looking at the fragments using an ionisation chamber. They coated one of the plates with a thin layer of uranium oxide, and they could immediately see a large number of small pulses from the alpha particles of uranium. But when exposed to the bombardment of neutrons from the cyclotron or Radon-Beryllium (Rn-Be) source very large pulses were detected.
The cyclotron was developed at Berkeley by Ernest O. Lawrence in the period 1931, and by 1936 John Ray Dunning and Herbert Lawrence Anderson had built one at Columbia University.

This is what people called the ‘atom smasher’. This machine produced an energetic beam of protons which then struck a stationary metal target to produce a secondary neutron beam. In this sense it was an alternative to the ‘natural’ Ra-Be neutron source. This machine was used to show that it was uranium-235 that was the most readily fissile component of uranium. What we see above is the core component of the cyclotron which was decommissioned in 1965 and given to the Smithsonian Institute for restoration and display. The other component of the cyclotron were the large electro-magnets. Below we have Dunning (left), Fermi (centre) and Mitchell (right) standing in front of the magnets housed in the basement of Pupin Hall in Columbia University.

In 2006 it was finally decided to remove the 30 ton magnets for scrap.

Below is a photograph of the large cyclotron at the Lawrence Radiation Laboratory, showing how the cyclotron cavity, etc. would be inserted between the poles of the electro-magnet.

Now back to fission fragments. Looking at the ratio of the pulses they saw in their ionisation chamber they were able to estimate that the energies of the fragments of uranium ranged up to about 90 MeV, and given that the expectation was a total energy release of about 200 MeV this suggested that there were two fragments one somewhat more energetic and the other less so. At the same time Frisch had cabled Bohr with the same results. Fermi moved onto the measurement of the ‘cross-section‘ of the fission process for neutrons of different energies. They coated the inside of the ionisation chamber with a very thin layer of uranium oxide, and bombarded it with a known number of neutrons from an Rn-Be source of known intensity placed inside a paraffin block. Thermal neutrons were isolated by measurements with and without a cadmium absorber. They estimated the cross-section of thermal neutron for the fission process as about 2 x 10-24 cm2 (compared to 0.1 x 10-24 cm2 for fast neutrons). Further experiments suggested that the efficiency of slow neutrons for the fission process followed an inverse law of the velocity (1/v). This appeared inconsistent with the known fact that in uranium there was a sharp resonance for slow neutrons at about 25 eV which did not lead to fission but to the formation of uranium-239. It was Bohr who suggested that the fission might not be occurring in uranium-238 but in uranium-235 (present in natural occurring uranium with a concentration of about 0.7%).

It is worthwhile mentioning here that neutrons are neutral particles that travel in straight lines, and are deviated only by collision with a nucleus (not with the electric field around the nucleus). They can be deviated or absorbed. The ‘barn‘ is a unit for measurements of cross-sections.
Above we have a very effective presentation of the meaning of ‘cross-section‘. It is an effective area that represents the likelihood of a certain type of interaction (a kind of collision). The cross-section of a nucleus represents the probability of a particular type of nuclear reaction, so it is in many ways a ‘characteristic target area’, but it can vary significantly depending upon the type and velocity of the ‘projectile’ (particle). The total cross-section is made up of several different types of interaction, i.e. elastic scattering (n,n), inelastic scattering (n,n’), radiative absorption (n,𝜸), and fission (n/f).

What Fermi was interested in was the possibility for neutrons to be absorbed and to subsequently emit other neutrons that would lead to a self-sustaining or ‘chain reaction’. The reality is that such a reaction cannot occur in natural uranium, and we can see this in the neutron cross-sections for uranium-235 and uranium-238. Uranium-235 will fission (n,f) at all energies (we call this a fissile material). Uranium-238 has a lower threshold for fission (n,f) at about 1 MeV. There is a very strong resonance capture of neutrons (n,𝜸) in the energy range 10-100 eV, in particular for uranium-238. The fission neutron energy spectrum peaks at around 1 MeV and at this energy the inelastic cross-section (n,n’) for uranium-238 exceeds the fission cross-section, essentially preventing fission for uranium-238. The fission cross-section is small for the fission neutrons and therefore the neutrons are absorbed by other processes, thus natural uranium cannot sustain a fission chain reaction.
The classical way to view this is to take 100 fission neutrons, 98 will be captured in uranium-238 and only 8 of those captured will result in a fission. The remaining 2 neutrons will cause fission in uranium-235, and each fission will produce 2-3 neutrons. So we started with 100 neutrons, and now in the second generation we have a maximum of 25, thus no chain reaction.
How to get around this? Firstly we can enrich the uranium with more uranium-235. A 50-50 mix will easily sustain a chain reaction with most of the fission events occurring in uranium-235 in the energy range 03-2.0 keV. This is called a ‘fast reactor‘. Or we can mix natural uranium with a material (a moderator) that slows down the neutrons without absorption, as we get to the low energies the fission cross-section of uranium-235 increases rapidly. Now the fissions are induced by thermal neutrons (0.025 eV), hence the name ‘thermal reactor‘.
The team at Columbia went on to use the cyclotron and collect the recoil fragments on Cellophane foils which were later measured for their activation. It was at this point that whilst the team pursued the details of the fission process, Fermi with Anderson focussed on neutron emission from the splitting of the uranium nucleus.
They knew neutrons were emitted in the process of fission, but did they emerge from the excited fragments or at the instant of fission? They started by placing the neutron source in the centre of a spherical bulb immersed in a large tank of water. They used the activation of rhodium foils placed at different locations in the tank to take measurements with and without uranium oxide inside the bulb. All the neutrons in the water are slow, and they found that there was a 6% increase with the uranium, corresponding to a yield of about 2 neutrons emitted for each neutron captured. These experiments took place independently of those of Hans Von Halban, Frédéric Joliot-Curie and Lew Kowarski in Paris.
What we tend to see is an almost ‘linear’ and highly logical picture of the key moments in the development of nuclear fission, and forget all the other experiments that are needed to understand the physics of the fission process. One of the problems with fission by slow neutrons is that uranium-238 can simply capture the neutron giving rise to the radioactive isotope uranium-239. This process competes with fission, thus absorbing neutrons that are needed to sustain a chain reaction. This appeared on the surface to be a simple question, but it turned out that neutron capture by uranium-238 leads, through neptunium, to the production of plutonium.
Meitner, Hahn and Strassmann had already published on the resonance capture process with a sharp absorption at about 25 eV (a cross-section of about 1200 x 10-24 cm2). But there was no information about the width of the absorption band or the cross-section for thermal neutrons. Fermi and Anderson found that the cross-section for the production of uranium-239 by thermal neutrons was only about 3.2 x 10-24 cm2 (it later turned out to be about 6 x 10-24 cm2) and the width of the resonance was determined at about 1 eV.
Resonance capture involves the creation of an excited state of a nucleus formed by the combination of the incident particle and the target nucleus. This occurs when the energy of the particle is near to a certain defined energy value (usually low energies). Different peaks correspond to different compound states of the nucleus.

Uranium-238 (above) is a fissionable isotope but not a fissile isotope. It is not capable of undergoing fission reactions after absorbing thermal neutrons. On the other hand it can be fissioned by fast neutrons with energies > 1 MeV. Uranium-238 is not able to sustain a nuclear fission chain reaction because too many of the neutrons produced by fission have lower energies than the origin neutron. We say that uranium-238 is a fertile isotope in that it’s radiative capture of a neutron leads to the formation of fissile plutonium-239.

Uranium-235 is a fissile isotope and it has a large fission cross-section for thermal neutrons, and a very small cross-section for fission with fast neutrons. Most of the absorption reactions result in fission, but about 15% are radiative capture, forming uranium-236.
About the same time Leo Szilárd was also in Columbia University. He had obtained about 20 kg of uranium oxide and they placed it around a Ra-Be source and all immersed in a bath of 10% manganese solution. This experiment confirmed that more neutrons were emitted by uranium than it absorbed. They went on to conclude that “a nuclear chain reaction could be maintained in a system in which neutrons are slowed down without much adsorption until they reach thermal energies and are then mostly absorbed by uranium rather than by another element“. There was an issue, in that in a system composed essentially of uranium and hydrogen (i.e. water) they would need to produce more neutrons from uranium than were absorbed by the uranium and hydrogen together. Too much hydrogen would prevent a chain reaction. So as of July 1939 their initial conclusion was that even with an ‘optimum’ concentration of hydrogen it was uncertain whether neutron production would exceed the total neutron absorption. At this time they thought that they would need to lump the uranium together to reduce the losses due to resonance absorption, and that the absorption of hydrogen in water would make it unusable for slowing down neutrons in a chain reaction.
Fermi and Szilárd did not get on that well, but it was Szilárd that became convinced that with graphite to slow down the neutrons the chain reaction was a certainty. He and Eugene Paul Wigner convinced Einstein to write his famous letter to President Roosevelt. Fermi and George Braxton Pegram (Dean of Columbia) had tried to alert the authorities to implications of atomic energy, but perhaps their language had been too cautious. In any case Einstein’s letter led in early 1940 to a first government grant of $6,000 for buying granite to determine its neutron absorption.
An interesting aside, Anderson’s doctorate thesis was on resonance absorption in uranium. Szilárd was concerned that uranium research should be kept a secret, and he wanted everyone to withhold publication. But he needed a first example, and Anderson’s thesis was that first example. He got his doctorate but the thesis was deposited and not published. Future authors in the field were also asked to withhold publication. Results funded by the U.S. government were marked ‘secret’.
With the $6,000 funding Szilárd was able to buy 1½ ton of graphite all nicely presented as bricks. The bricks were placed in a neat pile with holes left for inserting the rhodium foils for neutron activation. Rhodium activation has a half-life of 44 seconds, so the foils had to be pulled and measured quickly. Fermi loved the process of irradiating the foils, extracting them and rushing down the corridor, and watching the scaling circuit light up on the Geiger counter. The results were that graphite was the obvious choice to slow down neutrons. The basic idea was that fast neutrons collide many times with graphite nuclei, losing energy until they reach thermal energy. Then they diffuse through the graphite without losing further energy, and then they are finally absorbed. The neutrons are slowed down more slowly than in water, but once they reach thermal energies the neutrons diffuse longer and reach greater distances from the source. This meant that it would be easier to physically separate thermal neutrons from higher energy neutrons. The general model describing this neutron diffusion is called the ‘age theory’.
I hate to do this but the below diagram shows how Fermi ‘saw’ the neutron slowing down process (forget the maths). We see that a fast neutron will travel quite a distance in a quasi-straight line losing energy in a few collisions. As it becomes a thermalised neutron it will start to meander around travelling quite a distance but not in a straight line (it can even turn back on itself). Finally it will be absorbed.

Another topic that attracted Fermi, and forced him to become a radio-chemist for a short while, was the so-called ‘branching ratios‘ in the fission of uranium-235. A large number of radioactive series were found among the fission products of uranium, and the branching ratio is the probability that a specific radioactive series occurs. With this experiment Fermi and Anderson used the expertise of the radio-chemist Aristid von Grosse. They were back using the cyclotron and by that time they had determined that slow neutron fission took place in the uranium-235. As far as I can tell the technique was to create a kind of standard measurement configuration with a uranium solution inside a paraffin block placed in a fixed position near the cyclotron. The configuration was designed to ensure that only slow neutrons were producing fissions in the uranium-235. Then a known quantity of each element was added as a carrier to the uranium solution, and then after irradiation the solution was purified and separated. A weighted fraction of the added amount was painted onto a thin aluminium strip and was wrapped around a thin walled silver-glass counter. This enabled the team to make comparative measurements in a highly standardised way, and then to calculate the branching ratio, the fraction of fissions giving rise to a particular radioactive series. From what I can understand the key task was to separate the different elemental series studied, i.e. iodine, antimony, barium, and zirconium. I must admit after reading the original research papers I could not extract a clear set of results, however the measurements were later repeated in an extensive way in the Metallurgical Laboratory in the University of Chicago.

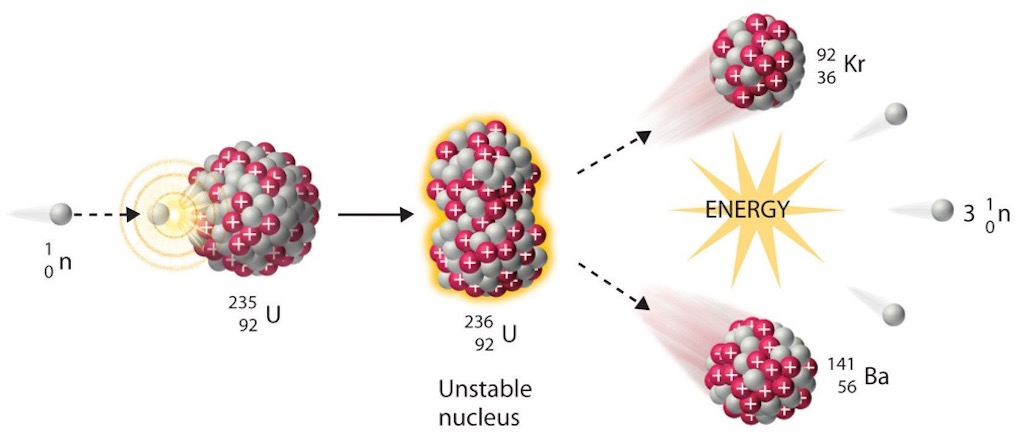
Above we can see the fission fragment yield for different nuclei. The most probable fragment masses are around krypton-95 and barium-137. For example as uranium-235 fissions the nucleus splits in to two smaller nuclei accompanied by a few neutrons. Most fission fragments are unstable and will decay. Most of the energy released in a fission is in the form of the kinetic energy of these fission fragments. As the fission fragments move through material they ionise the surrounding atoms, and gradually slow down. As the positive ions re-bond with the free electrons they release the excess energy in the form of heat.
Now that the Columbia team had a significant supply of graphite they could turn to the problem of precisely measuring the average number of neutrons produced by uranium upon the capture of a thermal neutron. Up to then the measurements were made with considerable uncertainty. They created a tall column of graphite with a Rn-Be source at one end and a window at about 70 cm from the source. The key was that at this distance the intensity of neutrons at a resonance energy was very low compared to the intensity of thermal neutrons. So uranium inserted in the window would absorb only thermal neutrons. After quite a complex set of side measurements, corrections, etc. the average number of neutrons produced per thermal neutron captured by uranium was 1.73.
All the above documented research focussed on understanding better the chain reaction as a way to produce power, not a bomb.
On 30 June, 1941 Fermi produced a kind of summary on the problems that might arise during the release of atomic energy. The starting point was that if a chain reaction was used as a radiation source, or as a way to create isotopes or produce new elements, then there would be a problem to dissipate the large amount of energy released during the reaction. In fact energy dissipation would probably be the limiting factor in how to exploit a chain reaction.
At that moment in time the idea was to use uranium without any separation of the isotopes, even if the separation of uranium-235 and of plutonium was already being studied and achieved albeit on a very small scale.
The basic idea was to assembly lumps of uranium metal or oxide in a lattice array throughout a mass of a light element (carbon, beryllium, heavy-water) used to slow the neutrons produced in the fission process and enable them to react again with the uranium reproducing new fission processes. The energy produced would be in the form of kinetic energy of the fission fragments, the beta- and gamma-rays, and the kinetic energy of the neutrons. All these energy sources will in the end produce heat, but it is not clear where this heat would be deposited (e.g. inside the materials or outside). Fermi estimated that most of the energy would be released by the kinetic energy of the fission fragments, and that most of the energy would be converted in to heat in the uranium lumps (perhaps as much as 90%). The reaction would need to be controlled to allow heat to be transported away from the lumps. More power could be generated if the heat was removed artificially. However most chemical elements that could be used to remove the heat have a high absorption for neutrons, and would likely stop the reaction. Probably the best way to remove heat is by using a fluid (gas or liquid) that would pass through channels or pipes. The heat could be used to vaporise the liquid, and the chain reaction would then become a boiler.
Gases could be helium or carbon dioxide (oxygen was ruled because of its poor chemical properties). Liquids could be deuterium based or possible liquid bismuth. Very large volumes of gases would be needed, liquid would require smaller volumes. Vaporised liquids in the form of heavy-water or some carbon fluorides had some promise.
The reaction would need to be controlled. However the change in the reaction could be exponential in both senses. The neutrons are emitted almost instantaneously and the speed in which the control would be applied would need to be of the order of 1/1000th of a second. Fortunately a reaction using delayed neutrons would require a speed of control of the order of 10 seconds. It may even be that the reaction is auto-controlled in that the rise in temperature might produce an increased loss of neutrons, and regulation could be achieved by managing the reflection properties of the materials.
Fermi concluded with the warning that the radiation emitted would be extremely dangerous, and a thick water screen would be needed.
It would appear that it was in spring 1941 that the military importance of the uranium work started to take precedence, despite the fact that most scientists doubted that atomic energy would come in time to affect the war. Some scientists such as Szilárd were convinced that an atomic bomb was feasible. In May 1941 there was a first cautious report on the difficulties in separating a sufficient amount of uranium-235 for a bomb. A report in July 1941 revealed the results on plutonium and mentioned the possibility of a bomb.
We are now in October 1941 and there was still a note of caution “separated uranium isotopes seem to bear out the assumption that fissions observed with neutrons of energy below 1 MeV are due to uranium-235“. Fermi’s calculation of the critical mass was 130 kg, but varied from 20 kg through to more than 1 ton. He also noted that the critical mass could be reduced by surrounding the ‘reacting unit’ with a reflector of very dense and think material. Nevertheless we are approaching the climax of the work in Columbia University, with a long series of experiments on different types of lattices of uranium oxide lumps embedded in graphite. Initially the tests were inconclusive, the purity of the uranium and the graphite was questioned, and the density of the oxide was considered too low. One particular problem was the leakage of neutrons from the structure, and the idea that a very large structure was needed. Fermi in his attempts to use a smaller structure, invented what they called an ‘exponential experiment’. This consisted of a rectangular column made up of a uranium-graphite lattice, and a neutron source placed at the bottom. The neutron density decreases exponentially along the length of the column, and is a function of the leakage along the column. A new grant of $40,000 allowed the team to build a lattice column about 2 metres square and nearly 4 metres high. With this they found a multiplication factor (or ‘reproduction factor ƙ‘) of 0.87, which despite being below 1 was seen as a very positive result. Everyone felt that by improving the purity, the geometry, and the density of the uranium they could exceed a multiplication factor of 1.
In fact at this moment in time no one appeared able to manufacture either non-pyrophoric uranium power (does not ignite spontaneously) or pure uranium ingots. In fact all isotopes of uranium are pyrophoric, but much depends upon the rate of surface oxidation balanced against the heat loss to the surroundings. However this feature is not the reason depleted uranium is used as kinetic energy penetrators. It is used because its density makes a bigger impact, and it is the abrupt slowing down upon impact that turns the penetrator white hot. In fact the tip is vaporised, and the projectile stays sharp because its alloyed with titanium forming a large metallic crystal. When it finally penetrates into the vehicle its a white-hot gas that ignites on-board ammunition and fuel. Plus depleted uranium is almost free.
In addition, at the time it was still problematic to obtain graphite with a low boron content. In fact it would take another 2 years to develop thermal and gas extraction purification techniques so as to obtain so-called nuclear-grade graphite. An order placed in May 1941 for 40 tons of graphite certainly helped.
In late 1941 Fermi made a masterful review of the chain reaction. The lattice consists of uranium lumps (at this time it was natural or un-enriched uranium) disposed in a mass of graphite. Fast neutrons are produced by the uranium lumps and are slowed down to thermal energies by collisions, primarily with carbon atoms. After reaching thermal energies the neutrons keep diffusing through the mass until absorbed by uranium or carbon. Some of the neutrons absorbed by the uranium atoms produce fission processes in which fast neutrons are emitted, and a new cycle of slowing down and absorption begins again. Fermi termed this a ‘generation’, and a key descriptor in the sequence of generations is the ‘reproduction factor ƙ‘ defined as “the average number of new fast neutrons produced in an infinite lattice by one original fast neutron in the first generation“. With ƙ>1 the series of generations expands exponentially. In a finite lattice ƙ is limited by the leakage of neutrons. Fermi calculated that a uranium lump would need to be surrounded by 327 cm of graphite for there to be no thermal neutron leakage.
Below I’ve included two diagrams of the uranium-235 chain reaction as we understand it today.


The team in Columbia then performed experiments to ascertain whether a lattice of uranium oxide lumps embedded in graphite could produce a ‘divergent’ chain reaction if the dimensions were sufficiently large. You will remember the uranium and graphite column. It was placed on a 3 metre square paraffin base about 30 cm high. A Ra-Be source was placed inside the paraffin base and intensity measurements were taken in different places in the column. It was in this experiment that a ƙ of 0.87 was found. They also found that ƙ was smaller at high temperatures than at low temperatures.
These were the last measurements made in Columbia University before the move to Chicago. The move was decided in January 1942. We have to remember that what had slowly emerged was the idea that a chain reaction could also be used to produce plutonium, as a competitor to uranium-235, for a possible atomic bomb. It was on the December 6, 1941 that it was decided to make an ‘all out’ effort on understanding the chain reaction. The next day Pearl Harbor was attacked, the U.S. declared war on Japan, Germany, and Italy, and Fermi became an enemy alien. It was under these circumstances that the Metallurgical Laboratory at the University of Chicago was established (February 1942). It was decided that they would study the chain reaction with natural uranium as a way to produce plutonium. Columbia University was already committed to two major projects, uranium separation headed by Harold Clayton Urey and the cyclotron project with John Ray Dunning. Fermi was an enemy alien, so Arthur Holly Compton was made project director for the work on the pile. He decided on Chicago, and Fermi and his team had to move. Fermi became submerged in an influx of scientists working on the chemistry of fission products and plutonium, on plant design, on the fabrication of uranium metal, and even specialists on the effect of radiation. And all this within am increasingly strict framework of military security.
We will continue to follow the work of Fermi and his team at Chicago in a later section dedicated to the Chicago Pile-1. In addition to the work going on in Columbia there were measurements being performed on alternative moderators to graphite. Samual King Allison was in the University of Chicago looking at beryllium as an alternative moderator, and Urey was still interested in heavy hydrogen (heavy-water) as another alternative moderator.
To close this parenthesis on Fermi in Columbia University on a lighter note. There are a number of stories about the tunnels under Columbia University, or as one writer put it “the abode of history, crime, and particle accelerators”. Over the years students had tried to investigate what was behind the ‘No Entry’ in the basement of the different buildings. There is even one tunnel that was used for transporting coal for the heating. And the tunnels were exploited by both the students and the police in the 1968 riots. As we have seen the basement of the Pupin Hall was home to one of the worlds first cyclotrons and was thus off-limits for radiation hazard reasons. As one writer noted, now the basement corridors have been refurbished and the traces of Fermi, et. al. have completely disappeared. Something to ponder on!