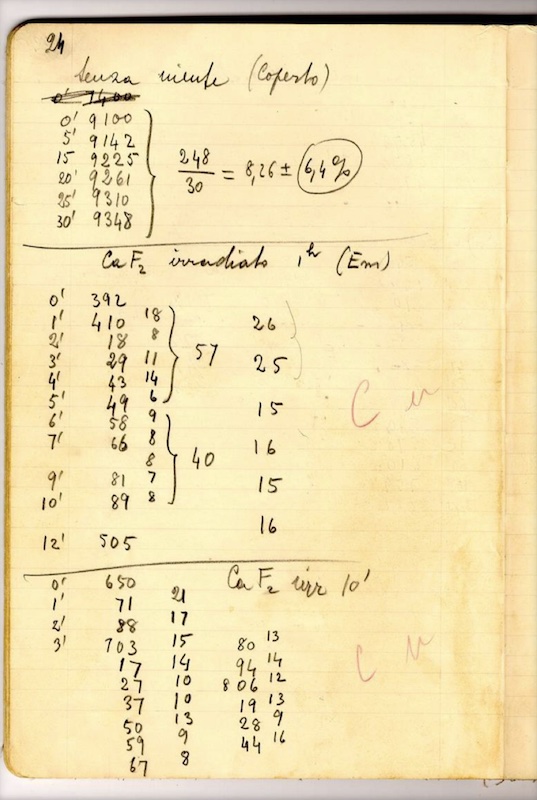
Introduction
The object of the project is to produce a practical military weapon in the form of a bomb….”
On July 13, 2011, U.S. Interior Secretary Kenneth Lee ‘Ken’ Salazar said “The secret development of the atomic bomb in multiple locations across the United States was an important story and one of the most transformative events in our nation’s history”.
He continued, “The Manhattan Project ushered in the atomic age, changed the role of the United States in the world community, and set the stage for the Cold War”.
The eight ‘Signature Facilities‘ that were part of the Manhattan Project were:
The Metallurgical Laboratory (‘Met Lab’), University of Chicago with the Chemistry Building and Chicago Pile (CP-1) site
X-10 Graphite Reactor, Oak Ridge, Tennessee
K-25 Gaseous-Diffusion Process Building, Oak Ridge
Y-12 Beta-3 Racetracks, Oak Ridge
B Reactor, Hanford, Washington
Chemical Separations Building (T Plant), Hanford
V-Site Assembly Building, Los Alamos, New Mexico
Trinity Site, Alamogordo, New Mexico.
Within each facility there were, what are called now ‘Signature Artefacts’, such as the Alpha and Beta calutron magnets and the small calutron in Y-12. Building 9204-3 (Beta 3), still has two Beta calutron racetracks (one still remains in standby today). These instruments or ‘tools’ are now considered historical artefacts, but what I want to capture in these pages is a little bit about what actually happened between 1939-1945.
As a starting point I have used here, more or less word-for-word, Chapters III and V of “Atomic Energy for Military Purposes” by H. D. Smyth. This report is subtitled “The Official Report on the Development of the Atomic Bomb” 1940-1945. Two very valuable additional reports were “The Manhattan Project” by Terrence R. Fehner and F. G. Gosling (2010), and “Manhattan: The Army and the Atomic Bomb” by Vincent C. Jones (1985). Another valuable and detail report which is publicly available is “A History of the United States Atomic Energy Commission: The New World 1939-1946“, by Richard G. Hewlett and Oscar E. Anderson, Jr. I have also started to use a blog called ‘Restricted Data‘ which I find particularly rich and interesting. Additions extracted from other sources are often presented in italics.
Using several different sources inevitably means that there are some overlapping references and some inconsistencies. In addition I have tried to extend the descriptions of the research done at the time, in particular in the field of nuclear physics.
The Manhattan Engineering District
The Manhattan Engineering District (MED, also known as the Manhattan Project) was initially headquartered in New York City. It had no prescribed territorial limits and functioned as a special ‘District’ (an U.S. Army term) for directing the atomic bomb project. Still today the U.S. Army Corps of Engineers uses ‘Division’ and ‘District’, with, for example, Southwestern Division in the U.S., Middle East District, and Europe District.
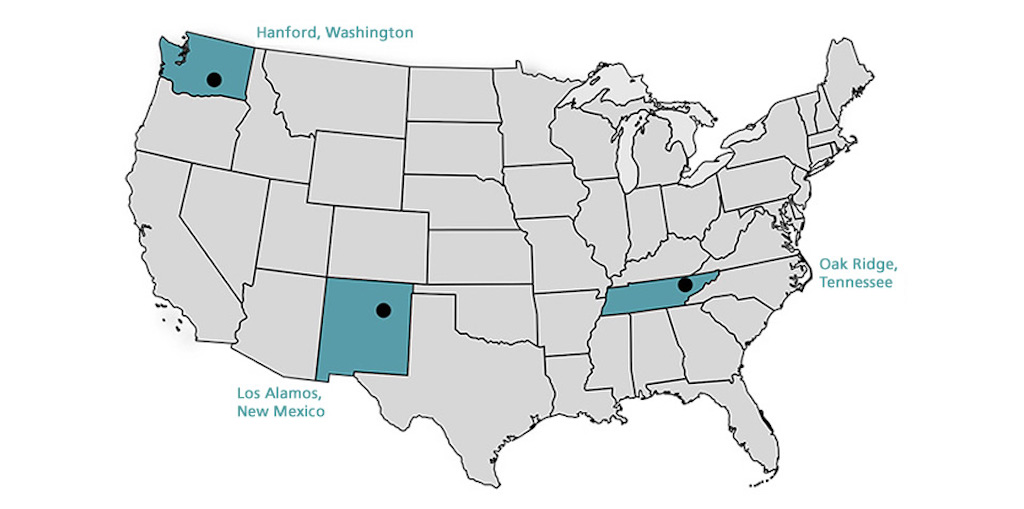
As such, the Manhattan Engineering District supervised research, development and testing, plant construction, and production programs relating to the project. The District also administered numerous laboratories and field installations, including the Clinton Engineer Works at Oak Ridge, Tenn., which performed separation of uranium isotopes; the Hanford Engineer Works at Richland, Wash., which produced plutonium; and the laboratory at Los Alamos, N. Mex., which performed final processing of the fissionable materials and assembled the finished atomic bombs. The District also maintained facilities in more than 30 cities and, during its peak operations, employed more than 129,000 people.
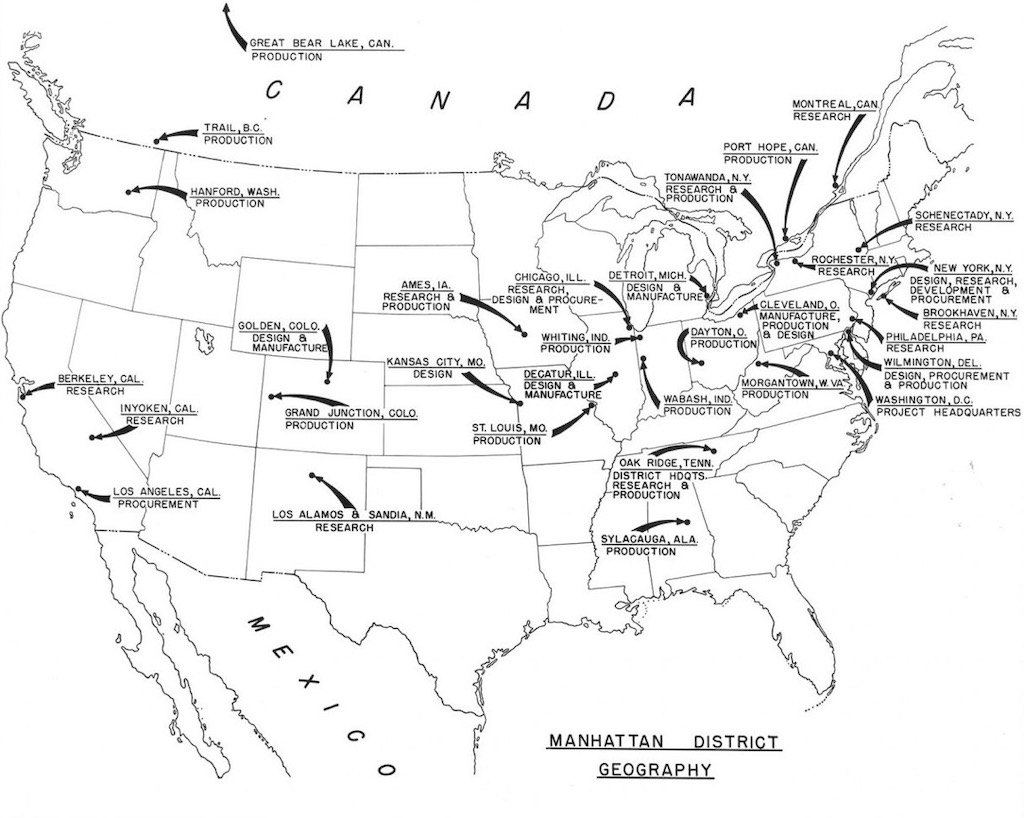
By the end of 1945, the overall cost of the Manhattan Project had reached nearly $2.2 billion, and in 1947 the annual running costs were $300 million. With the creation in 1946 of the AEC Atomic Energy Commission (as part of the Atomic Energy Act) the U.S. Army transferred to the AEC 254 military officers, 1,688 enlisted men, 3,950 government workers, 37,800 contract employees, and 37 installations in 19 different U.S. states and Canada.
At the end of the Manhattan Project its size exceeded the U.S. domestic automobile industry. If we just take one site, Oak Ridge, it was employing 12,000 people, and by 1945 it was consuming three times as much electricity as the city of Detroit. According to The Nuclear Security Blog the costs were repartitioned as indicated below, with Oak Ridge taking more than 50%.
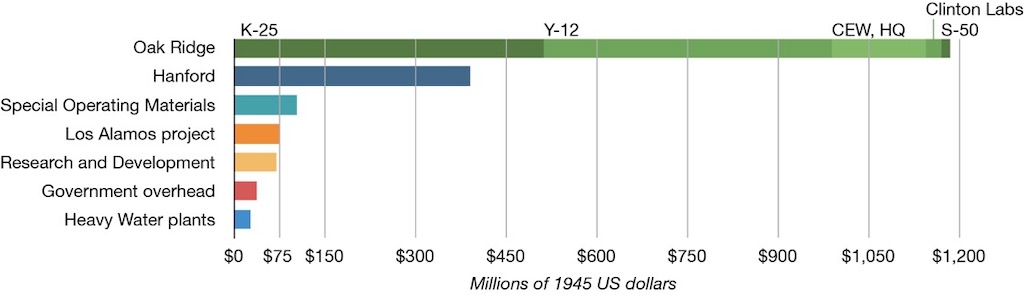
According to one estimate, the Manhattan Project cost $2.2 billion from 1942 to 1946 ($22 billion in 2008 dollars), which is much greater than the original cost estimate of approximately $148 million for 1942 to 1944. General Leslie Richard Groves Jr., who managed the Manhattan Project, has written that Members of Congress who inquired about the project were discouraged by the Secretary of War from asking questions or visiting sites. After the project was under way for over a year, in February 1944, War Department officials received essentially a “blank check” for the project from Congressional leadership who “remained completely in the dark” about the Manhattan Project, according to Groves and other experts.
In 2008 dollars, the cumulative cost of the Manhattan project over 5 fiscal years was approximately $22 billion, as compared to approximately $98 billion for the Apollo program over 14 fiscal years. A measure of the U.S. commitment to the programs was their relative shares of the federal outlays during the years of peak funding. For the Manhattan Project, the peak year funding was 1% of federal outlays, and for the Apollo program, 2.2%. Another measure of the commitment was their relative shares of the U.S. gross domestic product (GDP) during the peak years of funding. For both the Manhattan Project and the Apollo program, the peak year funding reached 0.4% of GDP.
General Leslie Richard Groves Jr. (1896-1970), oversaw the construction of the Pentagon and directed the Manhattan Engineering District (1942-1947). He later went on to be vice-president of Sperry Rand.
On July 16, 1945, the first atomic bomb was successfully detonated at Alamogordo, N. Mexico (more usually called the Trinity Site).
On August 6, 1945, and on August 9, 1945, atomic bombs were dropped on Hiroshima and Nagasaki, Japan, respectively.
During the summer of 1944, the problem of postwar control of nuclear energy began to receive serious consideration. In a memorandum of July 27, 1944, James Bryant Conant suggested the creation of a “Commission on Atomic Energy” to be charged with postwar development of nuclear energy for civilian and military purposes. Over the next few months, Conant, along with Vannevar Bush, urged the establishment of such a Commission.
James Bryant Conant (1983-1978), was a chemist, President of Harvard University, and first U.S. Ambassador to West Germany. He worked on poison gases for the U.S. Army in WW I, in particular lewisite. In 1941 he became chairman of the U.S. National Defense Research Committee (NDRC).
Vannevar Bush (1890-1974), was an engineer, and during WW II headed the U.S. Office of Scientific Research and Development. He founded Raytheon, was chairman of Merck, regent of the Smithsonian Institution, and “invented” memex, a proto-hypertext system. He was instrumental in creating the U.S. National Science Foundation.
On May 4, 1945, Henry Lewis Stimson appointed an Interim Committee “to survey and make recommendations on postwar research, development, and controls, as well as legislation necessary to effectuate them“. The committee was chaired by the Secretary of War and also included the U.S. Secretary of State James Francis Byrnes, Assistant Secretary of State William Lockhart Clayton, Undersecretary of the Navy Ralph Austin Bard, OSRD’s Karl Taylor Compton, Bush, and Conant. The work of the Interim Committee eventually resulted in the establishment of the U.S. Atomic Energy Commission (AEC) by the Atomic Energy Act of August 1, 1946. All phases of nuclear energy research and production came under the control of the AEC on January 1, 1947.
Henry Lewis Stimson (1867-1950) was the U.S. Secretary of War for both WW I and WW II.
On December 31, 1946, Harry S. Truman signed the executive order that transferred peacetime control of the program to the AEC. This marked the end of the Manhattan Project, and transferred ownership of 37 military installations to the civilian commission. The Army also provided the new supervisors of the nation’s nuclear program with a cadre of nearly 3,700 U.S. troops and 4,000 U.S. government civilian employees to provide continuity to the program, just as Groves had called for a year earlier.
At the end of the WW II the Manhattan Engineer District continued for about 6 months, solely as an administrative agency to close out the project and handle personnel assigned to the AEC during the transition period. On December 31, 1946, a joint Army-Navy organisation, the U.S. Armed Forces Special Weapons Project (AFSWP), was established to assume the military functions of the Manhattan Engineering District.
During WW I, Thomas Edison suggested “to the Navy that it should bring into the war effort at least one physicist in case it became necessary to calculate something”. The Manhattan project brought together 1,000’s of physicists, chemists, mathematicians, and engineers. After WW II the effect of science on our society has been incredible, physicists have now become ‘experts’ on almost everything, and today every government understands that science is a major element in asserting its national power.
How it All Started
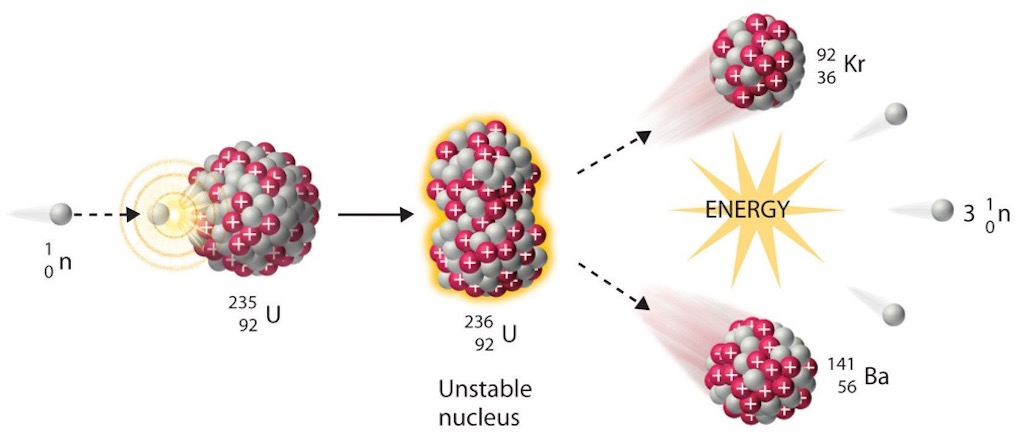
The announcement of the hypothesis of fission and its experimental confirmation took place in January 1939. There was immediate interest in the possible military use of the large amounts of energy released in fission. Beginning in 1939, some key scientists expressed concern that Germany might be building an atomic weapon. The early efforts both at restricting publication and at getting government support were stimulated largely by a small group of foreign-born physicists centred on Leo Szilárd and including Eugene Paul Wigner, Edward Teller, Victor Frederick Weisskopf, and Enrico Fermi. At the same time it was proposed that the United States accelerate atomic research in response to the perceived German threat.
The ‘Martians’, four brilliant scientists who were born in the same neighbourhood in Budapest, Hungary, allegedly earned their nickname from Enrico Fermi. A refugee from Italy himself, Fermi quipped that there must have been a spaceship from Mars that landed in Budapest, dropping off the extraordinarily gifted Edward Teller, Eugene Wigner, John von Neumann, and Leo Szilárd.
One of the remarkable things about the Manhattan Project was that so many “one in a generation” minds gathered at Los Alamos to collaborate on designing and building the bomb. The Manhattan Project boasted eight Nobel Prize laureates: Enrico Fermi, James Chadwick, Niels Henrik David Bohr, Arthur Holly Compton, Ernest Orlando Lawrence, James Franck, Harold Clayton Urey, and Isidor Isaac Rabi. After the war, over a dozen Manhattan Project veterans would go on to win Nobel Prizes, including Hans Albrecht Bethe, Emilio Gino Segré, Eugene Paul Wigner, Richard Phillips Feynman, and Glenn Theodore Seaborg. Then there were brilliant scientists, including Julius Robert Oppenheimer, Chien-Shiung Wu, and John von Neumann, whose valuable contributions were essential to the success of the project and changed science and mathematics forever.
Julius Robert Oppenheimer (1904-1967) was wartime head of Los Alamos, and is often called the “farther of the atomic bomb”. His achievements include the Born–Oppenheimer approximation for molecular wavefunctions, work on the theory of electrons and positrons, the Oppenheimer–Phillips process in nuclear fusion, the Tolman-Oppenhiemer-Volkoff limit for the mass of stars, and the first prediction of quantum tunneling. After WW II, he became director of the Institute for Advanced Study in Princeton.
In the spring of 1939 the group of Szilárd enlisted Niels Bohr’s cooperation in an attempt to stop publication of further data by voluntary agreement, but publication continued freely for about another year although a few papers were withheld voluntarily by their authors. At the April 1940 meeting of the Division of Physical Sciences of the U.S. National Research Council, Gregory Breit proposed forming a censorship committee to control publication in all American scientific journals. This arrangement was very successful in preventing publication and was still nominally in effect, in a modified form, through to June 1945.
We have to remember that the ‘modern’ science community of that time was a web of connections, both as friends and professionals. For example, Oppenheimer worked with Bohr, who was teaching Teller. Fellow Hungarian Szilárd met Wigner in 1921 while studying under Einstein, von Laue, and Planck in Berlin.
When in January 1939 at the “Fifth Washington Conference on Theoretical Physics: Low Temperature Physics and Superconductivity”, Niels Bohr delivered a lecture on splitting the nucleus of uranium, he aroused the concern of numerous conscientious scientists. Leo Szilárd, “obviously concerned, took [Edward Teller] aside“, and said, “Let’s be careful. Let’s not talk about this too much“. Teller agreed and “concentrated on returning the conference to the subject of low temperatures“. Szilárd, Teller and others were aware that, given the capabilities of German physics and the inclinations of Hitler, the world might be endangered by this scientific discovery.
Strictly speaking Bohr did not deliver a lecture on splitting the nucleus of uranium, what he made was a short unannounced intervention (interruption) at the conference. Attendees were amazed by the announcement. He revealed that Otto Hahn and Friedrich Wilhelm ‘Fritz’ Strassmann of the Kaiser Wilhelm Institute in Germany, and Lise Meitner with Otto Robert Frisch, both Austrian physicists in exile (Meitner in Sweden and Frisch in Denmark), had been successful in splitting the atom. Among the people sitting in that room were Fermi, Bohr, Urey, Rabi, Teller, Bethe, Roberts, and Johnson, all names we will encounter in these pages on the Manhattan Project.
The first contact with the U.S. government was made by George Brexton Pegram of Columbia University in March 1939. Pegram telephoned to the Navy Department and arranged for a conference between representatives of the Navy and Fermi. The only outcome of this conference was that the Navy expressed interest and asked to be kept informed.
George Brexton Pegram (1876-1958) brokered a meeting between Fermi and the U.S. Navy on the prospects of the atomic bomb. He was chair of the physics department in Columbia University and help found Brookhaven National Laboratory. Fermi, Rabi, Szilárd, Anderson, and Zinn all worked in the Columbia physics department.
The next attempt to interest the government was stimulated by Szilárd and Wigner. In July 1939 they conferred with Einstein, and a little later Einstein, Wigner, and Szilárd discussed the problem with Alexander Sachs of New York. In the fall Sachs, supported by a letter from Einstein, explained to President Roosevelt the desirability of encouraging work in this field.
Alexander Sachs (1893-1973) was Lehman Brothers chief economist, and friend of Roosevelt (and some called him also a pompous financier).
On October 11, 1939, Sachs met with the President to discuss the letter written by Albert Einstein the previous August. Einstein had written to inform Roosevelt that recent research on chain reactions utilising uranium made it probable that large amounts of power could be produced by a chain reaction and that, by harnessing this power, the construction of “extremely powerful bombs…” was conceivable. Einstein believed the German government was actively supporting research in this area and urged the United States government to do likewise. Sachs read from a cover letter he had prepared and briefed Roosevelt on the main points contained in Einstein’s letter. Initially the President was noncommittal and expressed concern over locating the necessary funds, but at a second meeting over breakfast the next morning Roosevelt became convinced of the value of exploring atomic energy.
Einstein drafted his famous letter with the help of the Hungarian emigre physicist Leo Szilárd, one of a number of European scientists who had fled to the United States in the 1930’s to escape Nazi and Fascist repression. Szilárd was among the most vocal of those advocating a program to develop bombs based on recent findings in nuclear physics and chemistry. Those like Szilárd and fellow Hungarian refugee physicists Edward Teller and Eugene Wigner regarded it as their responsibility to alert Americans to the possibility that German scientists might win the race to build an atomic bomb and to warn that Hitler would be more than willing to resort to such a weapon.
But Roosevelt, preoccupied with events in Europe, took over two months to meet with Sachs after receiving Einstein’s letter. Szilárd and his colleagues interpreted Roosevelt’s inaction as unwelcome evidence that the President did not take the threat of nuclear warfare seriously.
Roosevelt wrote back to Einstein on October 19, 1939, informing the physicist that he had setup a committee consisting of Sachs and representatives from the Army and Navy to study uranium. Events proved that the President was a man of considerable action once he had chosen a direction. In fact, Roosevelt’s approval of uranium research in October 1939, based on his belief that the United States could not take the risk of allowing Hitler to achieve unilateral possession of “extremely powerful bombs”, was merely the first decision among many that ultimately led to the establishment of the only atomic bomb effort that succeeded in WW II – the Manhattan Project.
The story of Einstein’s letter is that Szilárd was convinced that a uranium-graphite experiment might prove successful and it was both imperative to get on with the work and to take steps to keep the uranium ore of the Belgian Congo out of German hands. In talking with his friend Eugene Wigner they thought that Einstein, who knew the Belgian royal family, should write to them. Einstein actually dictated a letter of warning intended for someone of lower rank. But Wigner felt that when contacting a foreign government the U.S. Department of State should be informed. Szilárd felt that it was better still to make some form of direct contact with Washington. Gustav Stolper suggested contacting his friend Alexander Sachs who had ready access to the White House. By the time Szilárd approach Sachs, he had already pointed out to Roosevelt the importance of the work in nuclear physics. It was Sachs who suggested a letter from Einstein to Roosevelt.
Stepping Back in Time
Before looking at how the U.S. responded to Einstein’s famous letter, let’s look at how the ideas about nuclear fission evolved.
We have to start somewhere, and as good a place as any is in 1930 when Walter Wilhelm Georg Bothe and Herbert Becker bombarded beryllium, boron, fluorine and lithium with alpha-particles from polonium and detected an unusually penetrating radiation (Bothe had already started in 1926 investigating the transmutation of elements by alpha-particles). Bothe and Becker thought this radiation was gamma radiation because unlike alpha particles this radiation was not influenced by an electric field. After their experiments Bothe estimated the photon energy from the degree of absorption of the secondary electrons, but the energy of ‘beryllium radiation’ varied greatly according to the substance used as absorber.
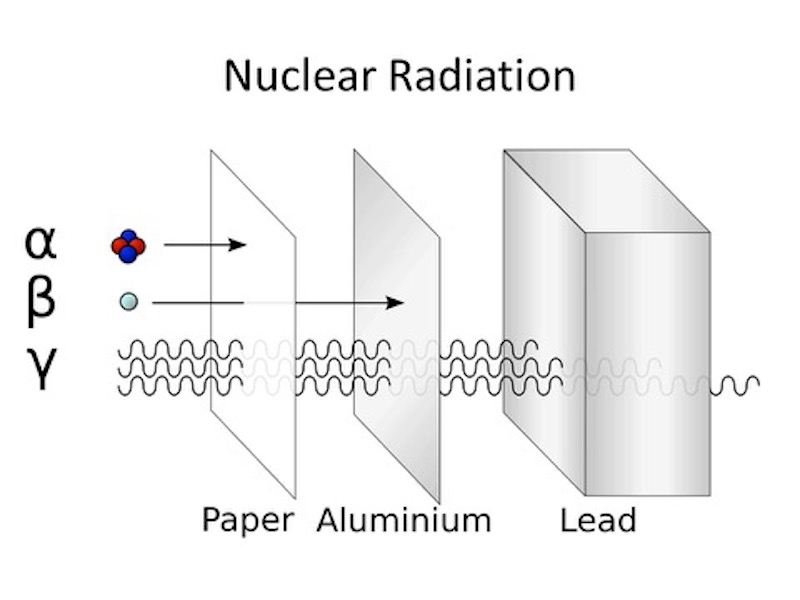
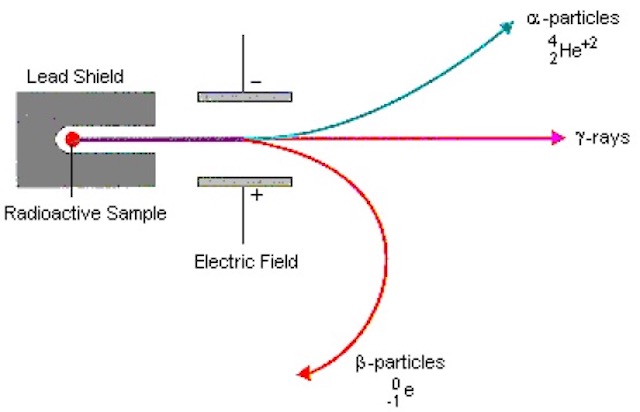
In Paris Irène Joliot-Curie and Frédéric Joliot found that this secondary radiation from beryllium and lithium penetrated through matter even easier than estimated initially by Bothe, and this new radiation passed through a far thicker layer of lead than gamma-rays. Although not inconsistent with gamma radiation, to produce a 5 MeV proton you would need a highly improbable 50 MeV gamma-ray, and at the time Ettore Majorana suggested that a new neutral particle was needed. They continued the study of the ionisation produced by secondary ‘beryllium radiation’. Using a ionisation chamber with a thin aluminium detector window they found that the signal increased when they placed hydrogen-containing compounds in front of the detector window. This was due to the ejection of high energy protons, and they continued to assumed that this ‘beryllium radiation’ was very high energy gamma-rays. They knew that the hypothesis was internally inconsistent, but they assumed that it was a “new mode of interaction of radiation with matter”.
Neither Ernest Rutherford not James Chadwick believed in the gamma-ray hypothesis. In fact Chadwick had studied with Johannes Wilhelm ‘Hans’ Geiger and Bothe at the Physikalisch Technische Reichsanstalt (now PTB) in Berlin. Both Chadwick and Geiger had studied under Rutherford, and Chadwick had been interned in the Ruhleben camp during WW I (Chadwick was helped during his internment by Max Planck, Walther Nernst and Lise Meitner).
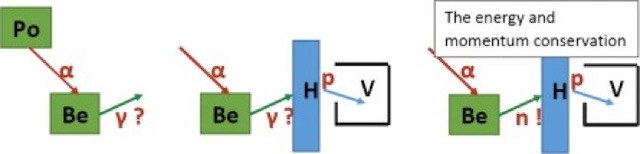
Above we have on the left the Bothe experiment where alpha-particles (in red) from the polonium emit a radiation (in green) which Bothe assumed were gamma-rays. In the middle we have the Joliot-Curie experiment where the hydrogen-based material (H) is hit by radiation (green) and emits high-energy protons. On the right we have Chadwick’s experiment where the neutron has now appeared and where energy and momentum are conserved.
We can see the wonderfully simplified presentation of the different experiments, but if we look below at the laboratory of Rutherford we can see that the reality of the time was significantly different.
It was in 1932 that Chadwick bombarded hydrogen and nitrogen with this new ‘radiation’ and from the recoil energies concluded that the mass of the new particle must be about equal to the that of a proton, but due to its penetrability it must be neutrally charged. He also demonstrated that the gamma-quanta hypothesis was in fact incompatible with energy and momentum conservation. The Joliot-Curies were not immediately convinced, but after additional experiments with a different reaction producing nitrogen they provided additional support for the neutron hypothesis. Chadwick was awarded the 1935 Nobel Prize in Physics for his discovery of the neutron.
Different teams of experimenters had remarked that placing hydrogen-containing materials (e.g. polyethylene) in front of a neutron detector produced a higher signal. The problem is that neutrons are thermalised in hydrogen-containing materials thus increasing the probability of interaction with the detector, but they also eject protons from hydrogen. So in any experiment you could have neutrons, gamma-quanta and protons, and the different type of detectors used by Bothe, the Curies and Chadwick all appeared to be selectively sensitive to different types of radiation. At the time all this made the interpretation more complex. And it must be said that many experimenters did not expect to observe neutrons, on the other hand people such as Chadwick were expecting to see them. Fermi had also noted that his experiments seemed to work better on a wooden table than a marble table, and at the time he suspected that the protons of the wood were slowing the neutrons and so increasing the chance for them to interact with nuclei. In fact his 1938 Nobel Prize for Physics explicitly noted “related discovery of nuclear reactions brought about by slow neutrons“.
The appearance of the neutron revolutionised the ‘classical’ proton-electron model of the atom, and quickly both Dimitri Ivanenko and Werner Heisenberg proposed new neutron-proton models of the atom. Heisenberg saw the neutron as a proton-electron composite, but he introduced the idea of nuclear exchange forces that bind nucleons and the idea that protons and neutrons were different quantum states of the same particle. Ivanenko proposed that atomic nuclei consisted only of protons and neutrons and he was also the first to propose the nuclear shell model, which later led to the postulate of a strong force to overcome electro-magnetic repulsion and bind the protons inside the atomic nucleus. Already in 1930 it was George Gamow that had proposed a semi-empirical liquid-drop model for the atomic nucleus. And it was in 1935 that the Bethe-Weiszsäcker formula was used to calculate the properties (including mass) of an atomic nucleus from its number of protons and neutrons.
From 1928 Irène Joliot-Curie and her husband Frédéric had studied atomic nuclei, but had failed to identify both the positron and the neutron (two missed opportunities for Nobel Prizes). However in 1934 they irradiated several light elements, including a natural stable isotope of aluminium (aluminium-27), with alpha particles from a polonium source and produced unstable isotopes of phosphorus (phosphorus-30). This opened the door to the quick and cheap production of radioisotopes (and third-time lucky for the 1935 Nobel Prize for Chemistry). And if you are attentive you will also have noticed that the creation of the ‘radio-phosphorus’ atom is accompanied by the emission of a neutron. Phosphorus-30 is unstable and in decaying to stable silicon-30 is a positron emitter with a 2.5 minute half-life. At the time Irène suggested that different nuclear reactions would produce other elements with other bombarding particles (protons, deuterons, and neutrons) but she also affirmed that nuclear fission was impossible.
When Enrico Fermi saw the 1934 results of Irène and Frédéric Joliot-Curie he immediately decided to switch to experimental neutron physics. He understood immediately that the neutron is unaffected by the Coulomb barrier so it could be used to induce nuclear reactions at lower energies and with all types of targets. This idea was key in that Fermi also saw the neutron not just as a product of a reaction, but also as an agent of a reaction.
These two lines are a kind of ‘official’ description, but the reality was somewhat different. The Physics Institute at the University of Rome ‘La Sapienza’ had produced some interesting theoretical work but had been unable to build a coherent body of experimental work. In 1932 already Fermi was debating whether to continue to ‘fool around’ with the Wilson cloud chamber, or to become (again) a theoretician, or to continue in the field of spectroscopy which was gradually being ‘worked out’. Finally Fermi with Franco Rasetti (two of the so-called ‘Via Panisperna’ boys, a name taken from the street where the laboratory was situated in Rome) decided on a research program in nuclear physics. Facilities were so poor in the laboratory that equipment building was ‘farmed out’, but they did manage to construct a large cloud chamber that worked perfectly. Their research budget at the time was about $2,000 to $3,000 per year, as compared to $200-500 a year in other Italian universities. Through 1933 the group continued to published important theoretical work, e.g. on nuclear spin, on neutron-proton interaction, on the theory of nuclei, and Fermi published his famous beta-decay theory (published in Italy and Germany, but refused by Nature as being “too speculative to be of any interest to the reader”).
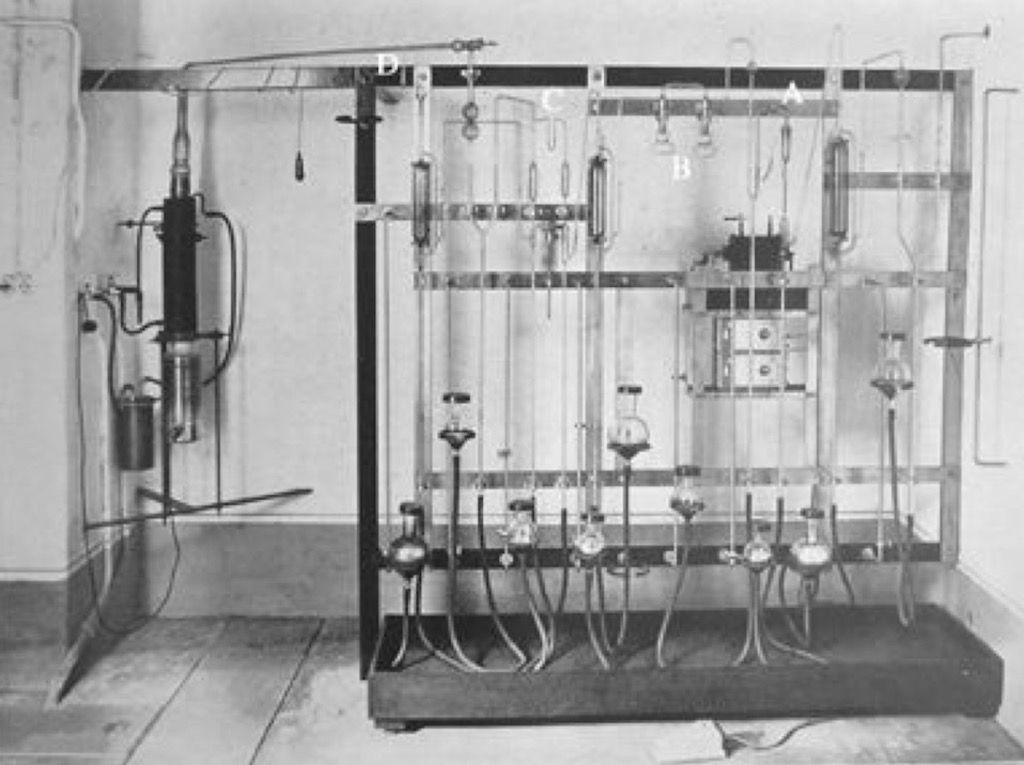
Often overlooked, a group that planned to do work in the nuclear field needed radioactive sources and detectors. This was not something that could be improvised. Obtaining radioactive substances was not an easy task. In 1933 Italian universities only had a few milligrams of radium salts, totally insufficient for a nuclear research program. On the other hand the neighbouring Institute of Public Health held more than 1.6 grams of radium salts for medical therapies (and 300 milligrams in reserve). The Physics Institute managed to get their hands on these radium salts and first extracted some radon (see the apparatus above) and more importantly made some polonium sources. As a pure alpha emitter polonium was the preferred research source at the time. As luck would have it to extract polonium the radium salts must not be fresh, and the salts held with the Health Institute were at least 14 years old. After learning the techniques in Kaiser Wilhelm Institut in Berlin and at Marie Curie‘s laboratories in Paris the team were able to extract about 110 mCi which at the time made it one of the worlds strongest radiation sources. With these sources Rome could start real experimental work in late 1933.
In parallel with the work on the sources, the team needed to acquire expertise in radiation detector systems. For this members of the team spent time in Florence and with Walther Bothe in Berlin. Below we have a typical ionisation chamber from the period, with the hollow cylinder for the specimen.

We can see now that when the discovery of artificial radioactivity was published in 1934, Fermi and his team in Rome were ready.
With Fermi it is always difficult to follow his work since when he was in Rome many of his interests overlapped and were intertwined. We have to think that during the early 1930’s experimental physics was focussed on looking at reactions producing particles of specific predictable energies. Theoreticians were not able to explain why a continuous spectrum of electrons were emitted during beta-decay. If beta-decay of a nucleus generated a single particle then energy conservation implied that the electrons must have a well-defined energy. But the observed energy spectrum was continuous, so did that mean that energy was not conserved?
Wolfgang Ernst Pauli had already suggested that another particle might be emitted simultaneously, but of course no one had seen this experimentally. But they would not see it if it were of very high energy, i.e. going through the detector with very high penetrating power and losing no energy that could be converted into a signal. Pauli actually joked about the particle, since it did not leave a signal he would not write about it. In 1933 Francis Perrin analysed the data on beta-decay and suggested a new particle (now called the neutrino) of almost zero mass and travelling at close the speed of light. Fermi quickly followed with his theory of beta-decay in which a neutron is transformed into a proton, an electron and an antineutrino. Essentially Fermi had invented the weak interaction.
So within a few years two new fundamental interactions (strong and weak) were added to gravity and electro-magnetism.
So now we have Fermi wanting to repeat the experiments of Joliot-Curie but with neutrons. Furthermore Fermi specifically thought that because of the general instability of heavy nuclei such as thorium and uranium they might see successive transformations.

His source was a beryllium powder mixed with radon in a small glass bulb (see above). The alpha particles from the radon hit the beryllium, and produce neutrons. A few months later he reported on the discovery of neutron-induced radioactivity on 22 different elements (a technique now known as neutron activation analysis). The Joliet-Curies went on to confirmed Fermi’s results for silver, silicon, zinc, iodine, and iron. From there ‘everyone’ started to discover other artificial transmutations so rapidly that within months there was a whole family of artificially produced radio-elements.
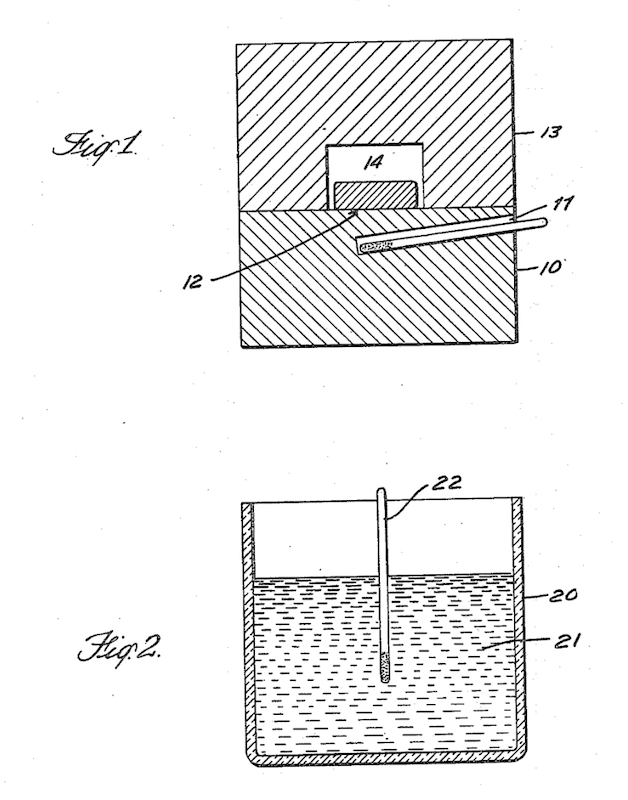
Above we can see Fermi’s original patent for the ‘production of radioactive substances’ where in Fig. 1 there is a cylindrical paraffin block (10) proved with a hole (11) in which a neutron source is inserted (this would be the radon and beryllium bulb). The material being irritated (12) is placed above the source on the paraffin block and is covered by a second paraffin block (13) having a central opening (14). As an example this would be a block about 24 cm in diameter, about 14 cm in height, with the neutron source about 2 cm under the upper surface. Fig. 2 is an alternative where the substance being irradiated is dissolved or dispersed in the energy reducing or dispersing material (21), in a container (20) with a neutron source placed in the middle (22).
Fermi also bombarded thorium and uranium with neutrons and (incorrectly) concluded that he had created two new elements, hesperium and audonien (see his notes below). Fermi had looked at a particular 13-minute decay period produced by neutron irradiation of uranium. Radiochemical tests had excluded that this radiation came from isotopes with a Z between 86 and 92, and therefore it appeared totally logical that the radiation must come from the element 93 or 94. At the time it was thought that fission was theoretically impossible, but they did expect elements with higher atomic numbers to be formed by neutron bombardment of lighter elements.
Rutherford wrote to Fermi on the April 23, 1934 congratulating him on his “successful escape from theoretical physics“.
Following Fermi’s publication there were a number of criticism as well as some erroneous confirmations. However the harshest criticism came from the chemist Ida Noddack who worked with her husband in the Physikalisch Technische Reichsanstalt in Berlin (they had previously discovered the element rhenium). Her argument was that it was insufficient to eliminate neighbouring elements of uranium and she suggested that the decaying nuclei might be “heavy fragments which are isotopes of known elements“. So the idea of nuclear fission was born on September 10, 1934, despite the fact that everyone thought the idea impossible. She was not able to provide any experimental data or theoretical model supporting her criticism, and did nothing to prove her hypothesis. Meitner and Frisch actually published a paper stating that the idea of fission fragments (not in those words) was rejected for “physical reasons” but the chemical evidence “was not entirely clear-cut“.
The work of Fermi was followed and reproduced by both the team in Berlin and in Paris. Irradiating uranium and thorium with both slow and fast neutron produces a multitude of nuclei/isotopes/isomers across a series of elements with similar chemical properties. But as the data came in so the internal contradictions became more evident. The problem became more evident, but not the solution. Meitner, Hahn and Strassmann did have doubts and they finally decided to disentangle all the new neutron-induced radioactivities. Physics up to WW II was a wonderfully open and international domain. Hahn had worked with William Ramsay in England in 1904, and it was Ramsey that convinced Hahn to take up radiochemistry and found him his first posting at the University of Berlin. But before moving to Berlin Hahn spent a few months with Rutherford in Montreal, then upon arriving in Berlin he met Lise Meitner in 1906 who had just arrived from working with Ludwig Boltzmann in Vienna. They joined forces to study radioactivity, and formed one of the most productive scientific partnership in the 20th C.
Lise Meitner was the second woman to obtain a degree in physics in Austria, and she was the first woman to ‘habilitieren’ in physics at the University of Berlin. Her first lecture in 1922 was enticed “On the importance of radioactivity on cosmic processes”, which the Berlin newspapers announced as a contribution on cosmetic physics. From September 1933, as a Jew, Meitner lost her teaching post and when Austria was annexed in March 1938 she automatically ‘became’ a German citizen. Fortunately Meitner left Berlin on July 14, 1938, otherwise she might not have survived the ‘Crystal Night‘ (November 9-10, 1938). Between 1938 and 1945 the Jewish population of Berlin fell from 170,000 to 5,000.
Over the years there had been considerable competition between Irène Joliot-Curie and Paul Savitch in Paris and the team Hahn, Meitner and Strassmann in Berlin. However they were all working on the idea that neutron irradiation of uranium would produce several ‘transuranium’ elements and a number of other products (still to be properly identified) through successive alpha decays. On November 8, 1938 Hahn and Strassmann published a paper claiming that the neutron irradiation of uranium would produce thorium-235 and radium-231 by successive alpha-decays. Bohr, Frisch and Meitner (in Stockholm at that time) told the authors that what they were proposing was unlikely purely on energetic considerations. So Hahn and Strassmann went back to the drawing board and decided to test what they thought was thorium and radium (and actinium as a transitory isotope), only to find that the three ‘fragments’ were in fact barium, lanthanum, and caesium. You have to imagine what this meant. How could a little thing like a neutron break in parts a heavy nucleus? And the worlds scientists were discussing the exact opposite process, the creation of heavier transuranium elements. The 1939 paper of Hahn and Strassmann clearly announced that nuclear fission had been discovered, but whilst the ‘solution’ was chemical, now physics would have to explain how a nucleus could split into two heavy fragments of about equal size.
On December 19, 1938 Meitner was the first to learn of the new results of Hahn and Strassmann, and Hahn asked her if she could explain what they had found (he actually asked for a ‘fantastic explanation’). Meitner was in Kungälv (north of Goteborg) spending New Year with her nephew Otto Robert Frisch. On January 16, 1939 Meitner and Frisch submitted a paper to Nature describing what the physical process might be. The idea was that the nucleus acts like a liquid drop, and with a violent impact could divide into two smaller drops. They even calculated the kinetic energy release to be about 200 MeV.
Frisch immediately realised that this release of energy must be easily measurable in an ionisation chamber. He returned to Copenhagen (where he worked with Bohr) and performed the experiment demonstrating that the kinetic energies were about 71 MeV per fragment. Thus nuclear fission was also physically confirmed.
Frisch told Bohr about the discovery and gave a first draft of their explanation of nuclear fission. Bohr left for America on January 7, 1939, travelling with Léon Rosenfeld (on the liner SS Drottningholm). On the January 16, 1939 Rosenfeld talked about the results at Princeton’s Journal club, and Bohr gave the news to a larger group of physicists at the Fifth Washington Conference on Theoretical Physics (January 26, 1939). The full story was published by the Physical Review on February 15, 1939.
By June 26, 1939 Niels Bohr and John Archibald Wheeler had published the basic theory of nuclear fission. In fact by the end of 1939 nearly 100 scientific articles had been published on the phenomena of fission.
An excess of the number of neutrons over the number of protons in a nucleus increases as a function of atomic weight, so it appeared natural that really heavy nuclei like uranium could emit not one but several neutrons. This would mean that one neutron ‘in’ could produce several neutrons ‘out’. This was a multiplying process, or as they said fission can run ‘in chain’. Multiple teams found that neutrons were liberated in the nuclear fission of uranium induced by slow neutron bombardment, and that the secondary neutrons had directions, energies, and temporal properties that were not directly related to the primary neutron process.
Already in his 1935 Nobel Prize lecture Frédéric Joliot-Curie had discussed the idea of a nuclear chain reaction and the important release of energy. In 1939 he and Hans von Halban, Lew Kowarski and Francis Perrin placed a source of neutrons in the centre of a copper sphere immersed in water. They filled the sphere with uranium oxide, or water, or a mixture, and measured the neutron fluxes in the various cases. This was to all intents and purposes a so-called sub-critical assembly. They were able to measure the mean number of neutrons produced per fission as 3.5+/-0.7 (today’s exact value is 2.41). This multiplicity established the possibility of nuclear chain reactions and thus nuclear energy production. The team even noted that had they made the sphere bigger fewer neutrons would have escaped. And the neutron multiplication factor would have increased until it exploded. Francis Perrin estimated this as the critical mass without a reflector around it.
These experiments used an external neutron flux to provide the initial fission events, but the model of Bohr and Wheeler predicted that a gamma-quanta could also initiate a chain reaction, and this was observed in 1940. At the same time Georgy Flyorov and Konstantin Petrzhak in Leningrad announced that a heavy enough nucleus could fission spontaneously, without an initial kick. This meant that a fission chain reaction could occur by itself once the conditions of critical mass were met.
Thought experiments abounded. You would need a low absorbing neutron moderator and reflector, like graphite or heavy-water. A neutron absorber (or ‘poison’), such as cadmium, could allow some kind of control so as to maintain a steady chain reaction. You could produce radioisotopes. You could produce energy. In May 1939 Joliot, Halban and Kowarski actually filed patents for power production and nuclear explosions. At the same time, from July 1938, there was an increasing exodus of Jewish scientists from mainland Europe to the United Kingdom and even more so to the U.S. By 1940 the whole topic of nuclear physics had become highly confidential.